Why does soap work so well on the SARS-CoV-2, the coronavirus and indeed most viruses? Because it is a self-assembled nanoparticle in which the weakest link is the lipid (fatty) bilayer. A two part thread about soap, viruses and supramolecular chemistry. v/ Palli Thordarson
[bit.ly]
The soap dissolves the fat membrane and the virus falls apart like a house of cards and "dies", or rather, we should say it becomes inactive as viruses aren’t really alive. Viruses can be active outside the body for hours, even days.
Disinfectants, or liquids, wipes, gels and creams containing alcohol (and soap) have a similar effects but are not really quite as good as normal soap. Apart from the alcohol and soap, the “antibacterial agents” in these products don't affect the virus structure much at all.
Consequently, many antibacterial products are basically just an expensive version of soap in terms of how they act on viruses. Soap is the best but alcohol wipes are good when soap is not practical or handy (e.g. office receptions).
[bit.ly]
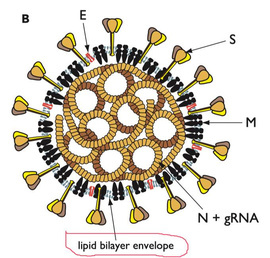
Most viruses consist of three key building blocks: RNA, proteins and lipids.
The RNA is the viral genetic material -it is very similar to DNA. The proteins have several roles including breaking into the target cell, assist with virus replication and basically to be a key building block (like a brick in a house) in the whole virus structure.
The lipids then form a coat around the virus, both for protection and to assist with its spread and cellular invasion. The RNA, proteins and lipids self-assemble to form the virus. Critically, there are no strong “covalent” bonds holding these units together.
Instead the viral self-assembly is based on weak “non-covalent” interactions between the proteins, RNA and lipids. Together these act together like a Velcro so it is very hard to break up the self-assembled viral particle. Still, we can do it (e.g. with soap!).
Most viruses, including the coronavirus, are between 50-200 nanometers – so they are truly nanoparticles. Nanoparticles have complex interactions with surfaces they are on. Same with viruses. Skin, steel, timber, fabric, paint and porcelain are very different surfaces.
When a virus invades a cell, the RNA “hijacks” the cellular machinery like a computer virus (!) and forces the cell to start to makes a lot of fresh copies of its own RNA and the various proteins that make up the virus.
These new RNA and protein molecules, self-assemble with lipids (usually readily present in the cell) to form new copies of the virus. That is, the virus does not photocopy itself, it makes copies of the building blocks which then self-assemble into new viruses!
All those new viruses eventually overwhelm the cell and it dies/explodes releasing viruses which then go on to infect more cells. In the lungs, some of these viruses end up in the airways and the mucous membranes surrounding these.
When you cough, or especially when you sneeze, tiny droplets from the airways can fly up to 10 meters (30 ft)! The larger ones are thought to be main coronavirus carriers and they can go at least 2 m (7 ft). Thus – cover your coughs & sneezes people!
These tiny droplets end on surfaces and often dry out quickly. But the viruses are still active! What happens next is all about supramolecular chemistry and how self-assembled nanoparticles (like the viruses) interact with their environment!
Now it is time to introduce a powerful supramolecular chemistry concept that effectively says: similar molecules appear to interact more strongly with each other than dissimilar ones. Wood, fabric and not to mention skin interact fairly strongly with viruses.
Contrast this with steel, porcelain and at least some plastics, e.g. teflon. The surface structure also matter – the flatter the surface the less the virus will “stick” to the surface. Rougher surfaces can actually pull the virus apart.
So why are surfaces different? The virus is held together by a combination of hydrogen bonds (like those in water) and what we call hydrophilic or “fat-like” interactions. The surface of fibres or wood for instance can form a lot of hydrogen bonds with the virus.
In contrast steel, porcelain or teflon do not form a lot of hydrogen bond with the virus. So the virus is not strongly bound to these surfaces. The virus is quite stable on these surface whereas it doesn’t stay active for as long on say fabric or wood.
For how long does the virus stay active? It depends. The SARS-CoV-2 coronavirus is thought to stay active on favourable surfaces for hours, possibly a day. Moisture (“dissolves&rdquo
The skin is an ideal surface for a virus! It is “organic” and the proteins and fatty acids in the dead cells on the surface interact with the virus through both hydrogen bonds and the “fat-like” hydrophilic interactions.
So when you touch say a steel surface with a virus particle on it, it will stick to your skin and hence get transferred onto your hands. But you are not (yet) infected. If you touch your face though, the virus can get transferred from your hands and on to your face.
And now the virus is dangerously close to the airways and the mucus type membranes in and around your mouth and eyes. So the virus can get in…and voila! You are infected (that is, unless your immune system kills the virus).
If the virus is on your hands you can pass it on by shaking someone’s else hand. Kisses, well, that's pretty obvious…It comes without saying that if someone sneezes right in your face you are kind of stuffed.
Posted on fb by Cecile G Tabura

Enjoy being online again!
Welcome to the community of good people who base their values on evidence and appreciate civil discourse - the social network you will enjoy.Create your free account
3 comments
Feel free to reply to any comment by clicking the "Reply" button.Plain water works if you use enough of it!
Soap and water just separate the oils from the surface of object!!!
When any virus find warm organic materials it can enter, the probability it will they to reproduce to infect it.
In other words if the droplets find an organic host before it dies, it will try to inter the host to infect it!!!
If you remove the lipid bilayer, you also remove the spike proteins REQUIRED for entry into the cell. This attachment and entry is receptor mediated, meaning not just any organic material will do, but only cells expressing the correct receptor in the right conformation for binding to the virus surface protein will suit. But yes, soap works to disrupt infection.
Enjoy being online again!
Welcome to the community of good people who base their values on evidence and appreciate civil discourse - the social network you will enjoy.Create your free account
Share this post
Categories
Agnostic does not evaluate or guarantee the accuracy of any content. Read full disclaimer.